Aspartame has been in the news again. Pepsico, which dropped aspartame from its diet Pepsi formulation over a year ago has reintroduced it. The motivation to remove it was driven by the bad press aspartame had received over the years which was thought to have impacted sales. It turns out that the aspartame version has a loyal following which persuaded Pepsico to backtrack. For more on what the controversy is all about…
What is Aspartame?
Aspartame is an artificial sweetener that was discovered in the 1960’s. It is approximately 200 times as sweet as an equivalent weight of sucrose (table sugar). It differs from all other artificial sweeteners in that it is made from two essential amino acids which are in turn important to a balanced diet. This is unusual since amino acids are normally associated with proteins which are not known for their sweetness (think steak, fish, etc.).
Aspartame, is considered to most closely match the taste of natural sugar. It is stable over a fairly wide pH and temperature range, However, at higher temperatures such as those used in baking, aspartame will breakdown and lose its sweetness.
Aspartame, Wrongly Accused Health Menace?
Unfortunately, the pseudoscience/pseudomedicine crowd decided to attack its safety with the same flawed reasoning as used with vaccines.
The infographic below lists the various products that aspartame yields when it breaks down. These include the amino acids aspartic acid and phenylalanine as well as methanol (aka methyl alcohol or wood alcohol).

Some of the chemistry behind aspartame is explained including the breakdown products and the extremely low risk they represent to our health. Infographic courtesy of Andy Brunning of Compoundchem.com.
Aspartame is one of the most studied molecules and is considered to be as safe or safer than other artificial sweeteners. One would have to drink 7.5 litres of diet soft drink to exceed the recommended safe level. Anyone who has ever tried to drink 8 glasses of water a day (2 litres), can appreciate that this is next to impossible.
Further, typical servings of wine and tomato juice have greater amounts of methanol than one can of soft drink.
Aspartame for the Biology Teacher
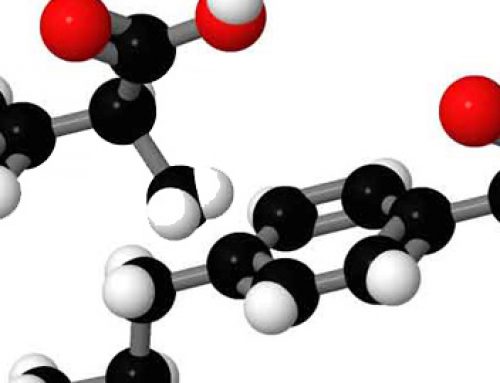
Aspartame, dipeptide, has the characteristic peptide bond that connects amino acids and is a good example of how VSEPR theory, a chemical principle, is relevant to biochemistry. In the peptide bond nitrogen changes from a tetrahedral to a trigonal pyramidal shape.

Aspartame is shown in our largest (Unit) and smaller (Molymod) molecular model styles. The Unit version is nearly 1 metre long and is a highly visible classroom demonstration tool.
The Molymod and Orbit (the smallest) versions can be shown and the parts needed to build them can be demonstrated with our 3D Molecular Model Builder. You can see a larger version of aspartame in Molymod.
The Bottom Line
Some studies have shown that any artificial sweetener may produce unwanted side effects. The trade off between this and the deleterious effects of obesity and diabetes need further research. Regardless, it appears that from a safety standpoint, aspartame is no worse and may be among the safest sugar substitutes.