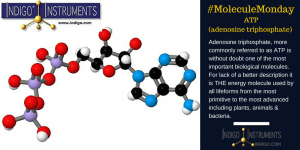
A little about Adenosine Triphosphate (ATP)
ATP was first discovered in 1929 by Karl Lohmann. Back then, it was considered that Adenosine Triphosphate (ATP) was an energy source produced inside the mitochondria of eukaryotic cells. Several years later in 1962, Geoffrey Burnstock discovered that it had the potential to carry messages between cells and that it helps in fighting human diseases. It was later concluded that ATP has a chemical molecular energy system that stores and releases energy at the same time.
For decades, biologists have been investigating exactly how the biological mechanisms work for Adenosine Triphosphate molecules. Particularly, in determining how ATP molecules move around inside cells and how the molecular signals turn on and off.
How does ATP Work?
ATP is composed of the nitrogenous base adenine, the five-carbon sugar ribose, and three phosphate groups. Energy is released when it loses a phosphate group as it goes from ATP it becomes ADP or Adenosine Diphosphate. Just the breaking of this one bond and the accompanying rearrangement is sufficient to liberate about 7.3 kilocalories per mole = 30.6 kJ/mol. This process is showcased in cellular respiration, the cycle which occurs in cells to convert nutrients to energy and waste. The “opposite” of this occurs in photosynthesis in which plants produce ATP.
Conclusion
According to Sunyoung Kim, a biological chemist, he explains that “ATP is the fuel of life. It’s an energy currency molecule — the most important source of chemical and mechanical energy in living systems”.
Therefore, ATP plays a vital role in the biological world. All living cells use Adenosine Triphosphate molecules as a fuel of energy to survive. Even viruses rely on ATP.
Make your own molecular model of Adenosine Triphosphate or any of the other chemicals mentioned on this page using our 3D Molecular Model Builder.