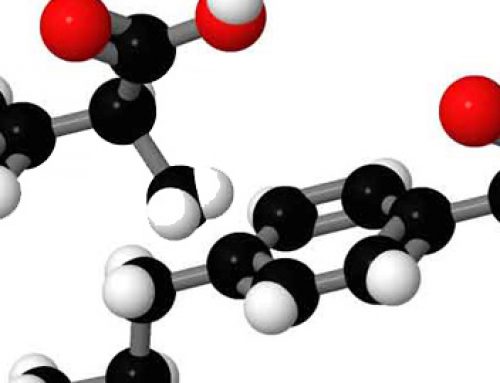
PABA or para aminobenzoic acid was one of the first chemicals used to prevent sunburn. It was added to sunscreens in order to block ultraviolet wavelengths. It shares chemical characteristics with other molecules such as melanin and chlorophyll and sunglass coatings.
How Does PABA Work?
PABA’s chemical makeup allows it to dissipate the energy of ultraviolet light. UV light has more energy in each photon than visible light. This higher energy can breaks rather than “bends” chemical bonds. This breakage in turn creates free radicals which can damage eyes & skin & DNA. It is these free radicals that antioxidants are supposed to inhibit. So, think of PABA as a pre-antioxidant.
The process is somewhat like photosynthesis. Chlorophyll absorbs certain wavelengths of sunlight & uses this energy to build molecules that sustain the plant and eventually the food we eat. PABA and chemicals like it also transfer energy but ultimate dissipate it as heat.
Some Other Examples
The same process occurs in sunglasses coated with UV protectors. Because you wouldn’t want these chemicals to come off in rain, they are applied differently but the principle is the same. Click on the infographic below for more information.

The chemicals used to block UV wavelengths in both sunscreens and sunglasses to prevent skin or eye damage share some features; in particular the number of double bonds.
Image used courtesy of Andy Brunning of Compound Interest.
PABA the Molecule
PABA, chlorophyll, melanin & other chemicals that absorb sunlight & transfer its energy all have one thing in common- ring structures with alternating single and double bonds. This construction allows for absorbed energy to move through the molecule. From there, the energy goes to produce new molecules or is released as heat.