Some elements you never heard of were given new names this week. Elements 113, 115, 117 and 118, were named nihonium, moscovium, tennessine and oganesson respectively. These elements were all synthesized in carefully designed experiments using sophisticated particle accelerators.
You’ll never see them on food labels or traded as commodity metals. In fact, they are so short lived they last just long enough for scientists to confirm they briefly existed.
Does that mean the miraculous metals of science fiction are impossble? Will there ever be a Vibranium of Captain America’s shield or the Duranium and Tritanium of Star Trek’s Enterprise? Will science find a way to make something like them? First, a brief survey of the elements we know and how they came to be.
In The Beginning
Shortly after the Big Bang nearly 14 billions years ago, the early universe consisted of hydrogen and much smaller amounts of helium and lithium. Gravity eventually caused these elements to coalesce into stars nd galaxies. Stars shine because gravity triggers nuclear fusion which mainly combines 2 hydrogen atoms to form a helium atom and releases tremendous amounts of energy. A small portion of this we see as visible light.
The Main Sequence Elements
As stars age and run short of hydrogen nuclear fusion shifts to “burning” helium. Eventually helium starts to run low and this processes continues until iron is the final element. This applies to the majority of stars including the Sun. The process is somewhat more complicated than this, you can read more at stellar evolution.
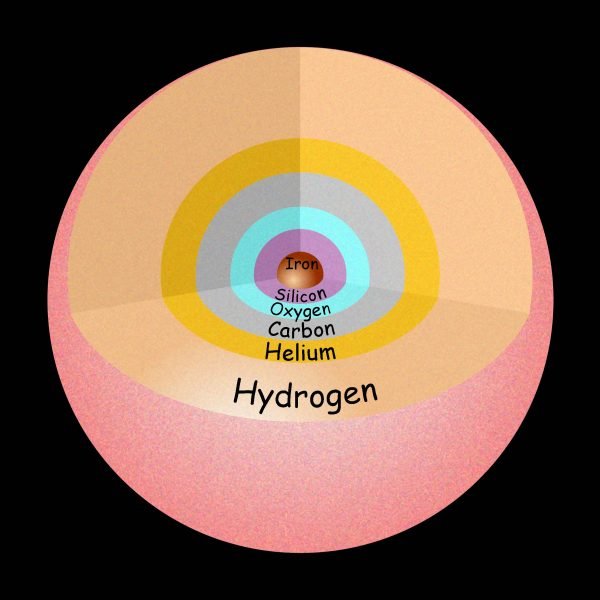
This picture shows some of the elements that are produced by nuclear fusion in stars like our Sun. The heaviest element is Iron (Fe).
Beyond the “Iron Curtain”
Anyone familiar with the periodic table, however, knows that planet Earth has elements beyond iron. As you can see in an image of the periodic table below, minerals containing elements as heavy as uranium are routinely mined. Where did they come from?

This version of the periodic table shows the naturally occurring elements up to Uranium (92). In the same row (red) you can see Americium used in smoke detectors, Technetium used in nuclear medicine and Plutonium, used in nuclear weapons.
The answer is that most of the heavier elements were created in supernovas, the end of life explosions of stars much larger than the Sun. The explosion scatters these heavier elements which go on to seed other gaseous clouds which give rise to new solar systems. While most were created that way, it appears that gold, platinum, uranium and a few others were formed in collisions between neutron stars. (1)
An Earth with all the elements we have today would not have been possible in the early evolution of the universe and may only been possible in the recent 4-5 billion years.
It was believed that elements beyond Uranium would never be found on Earth because of their relatively scarcity to begin with and they fairly short half-lives. As it happened, a tiny amount of plutonium was found in some rocks in 1971. However, all elements beyond Uranium have been made artificially.
Super Heavy Metal-A Dream?
50 years ago, if you knew what a white dwarf, red giant and a supernova were, you knew almost as much as the astronomers of the day. Since then, a whole spectrum of strange stellar phenomena have been discovered, from neutron stars to ultranovas to black holes.
Perhaps future discoveries will reveal mechanisms that can create heavier elements in the “Island of Stability“. If so, the miraculous materials of Iron Man’s suit, Captain America’s shield and the Enterprise’s hull may yet be possible.
Until then, we’ll have to be satisfied with the marvelous materials we didn’t have 50 years ago, Kevlar bullet proof vests or carbon fibre aircraft to name a few.
References
- Scientific American, June 2016: Stellar Fireworks.