What is Ammonia?
Ammonia, also known as anhydrous ammonia, is a simple inorganic molecule with the formula NH3. Along with methane, it contributed to the formation of amino acids, fatty acids and nucleic acids (AT & GC), the building blocks of life. Aside from ammonia’s chemical properties, its physical properties include low molecular weight (17.031 vs water 18.015) & a small kinetic diameter. Read more below for a discussion of its history & uses.

Ammonia (NH3) is a simple molecule consisting of nitrogen & hydrogen.
Some Ammonia Uses
Ammonia’s economic importance grew in the early 1900’s with the discovery of the Haber-Bosch process, a method of nitrogen fixation. Worldwide annual production of ammonia is around 200 million tons. It finds its way into an extraordinary list of products. From precursor in nitrogen-based fertilizers to explosives to household cleaners and disinfectants. It is an effective refrigerant for industrial freezers and hockey rinks and does less damage to the ozone layer than chlorofluorocarbons. Its very low molecular weight is especially useful in leak detection.
If you have an interest in chemistry, you can view examples of nitrogen in functional groups important in organic chemistry including amines, amides and nitriles.
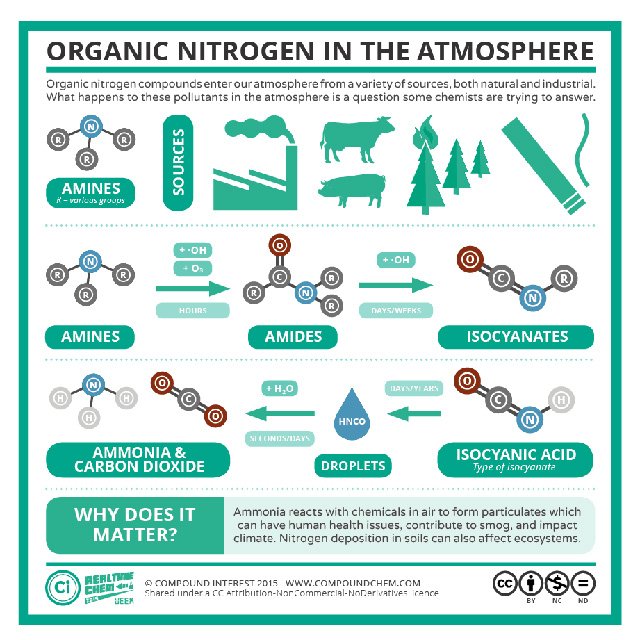
Atmospheric nitrogen is converted to ammonia, a feedstock for many important chemicals. Image courtesy of Andy Brunning at Compound Chemistry (https://www.compoundchem.com/).
Ammonia Refrigerant Leak Detection
Ammonia is an energy efficient & cost effective refrigerant. This makes it popular in large scale refrigeration units in places as diverse as food and beverage companies to ice hockey rinks. Ammonia has a low environmental impact compared to chlorofluorocarbons. Its pungent odor is distinctive & easy to smell even in low concentrations. However, it can be toxic in high concentration & prolonged exposure can be lethal. Confirming & locating leaks is essential.
Phenolphthalein is a chemical indicator that changes from colorless to pink/fuchsia in the presence of ammonia. The image below shows phenolphthalein papers that were dipped into test solutions calibrated at pH 9, 10 & 11. Concentrated ammonia has a pH of about 11.3 but at this high level of alkalinity, ammonia actually causes phenolphthalein to turn colorless. This has its advantages. If you see the paper reacting to produce the pink color it confirm that ammonia is present but not yet at toxic levels.
Test papers need to be wetted so the ammonia will be absorbed by the paper & react with the phenolphthalein. Distilled water is ideal but tap water works in a pinch. Gas line fittings are the best place to look first. The test result is only qualitative. i.e. a yes or no. However, the speed of the color change is proportional to the leak rate. If you can smell the ammonia but the test paper quickly goes from a pinkish color to colorless, it’s time to either get out or put on your respirator.

Phenolphthalein is a chemical indicator that changes pH in the presence of ammonia & is useful for the detection of ammonia leaks in refrigeration systems.
pH Chemical Indicators for Ammonia
Phenolphthalein is shown below. It has a very narrow range of pH response but when added to filter paper it is an economical method for finding ammonia leaks.
Phenolphthalein is a pH indicator made from the elements carbon, oxygen & hydrogen.
Bromothymol Blue
Bromothymol blue is also a pH indicator & reacts to ammonia as well. Its advantage over phenolphthalein is that shows its strongest response to high concentrations of ammonia.

Bromothymol blue is composed of carbon, oxygen, hydrogen, sulfur & bromine. Cloth infused with BTB responds to high concentrations of ammonia to indicate very tiny leaks.
Ammonia Leak Detection Cloth
The ammonia molecule’s size allows it to get through holes smaller than water & it is much cheaper to detect than helium. Cloth infused with bromothymol blue takes full advantage of the very small molecular size of ammonia to indicate the presence of leaks otherwise too small size to find. The cloth is much more durable than paper & can be reused multiple times.

40×40″ (1 sq. m. ) ammonia leak detection cloth costs roughly 1/3 per unit area of smaller 20″ versions. Cut it into smaller squares or rectangles or even into long strips for wrapping gas lines.
Liquid ammonia is readily available, inexpensive, easy to clean up and the indicator chemicals in the cloth are very sensitive. Ammonia leak cloth works on the principle that the cloth will turn from yellow-orange to blue in the presence of a very small amount of ammonia gas. This is reversible. The cloth reverts back to the original yellow-orange color in the absence of ammonia.

The bromothymol blue infused into cloth changes color when exposed to ammonia gas. Although the entire cloth has changed, in practice very tiny spots will react & identify leak locations.
Leak Detection in Isolators and Gloveboxes
Ammonia leak detection cloth can be used to detect leaks in equipment designed to be sealed or air-tight, such as incubators, pharmaceutical isolators, glove boxes, etc. A July 2012 article published in the journal Clean Air and Environment1 discusses the difference between leak rate and leak detection and the ISO classification of leak tightness based on volume loss per hour2, 3. It describes methods for conducting leak detection including ammonia leak cloth.

Scientists, engineers & technicians often need to handle dangerous materials that are radioactive, chemically toxic or biologically active material such as bacteria, fungi and viruses. Air tight sealed glove boxes provide a safe method for working with hazardous matter.
References
1) Coles, Tim (2012). Leak Rate Measurement for Pharmaceutical Isolators: Practical Guidance for Operators and Test Engineers. Clean Air and Containment Review Issue 11, pages 8-12.
2) ISO Standard 10648-2:1994. Containment enclosures—Part 2: Classification according to leak tightness and associated checking methods.
3) ISO Standard 14644-7:2004 Cleanroom and associated controlled environments—Part 7: Separative devices (clean air hoods, gloveboxes, isolators and mini-environments).





How much for test papers colored?
Click on Phenolphthein test papers if that is what you are looking for.