Star Trek fans will recall the episode “Devil in the Dark” where a silicon-based creature burrowed through rock. While we know of no such animal, silicon does form a diverse range of molecules. Here on Earth, it forms over 3000 different compounds and can be found in silicates such as beach sand, computer chips and clays. Silicon also forms aluminosilicates also known as zeolites which are used as oil cracking catalysts, water purifiers & other important applications.
If a well presented argument against the chances of silicon life interests you, go right to Reference #2 at the bottom of this page.
What is a Zeolite?
Zeolites are a class of natural and synthetic silicate compounds characterized by “cage” structures. Their chemical composition is mainly silicon and oxygen with varying amounts of aluminum substituting for silicon. Other elements such as sodium, calcium, and magnesium also contribute to hundreds of different versions. Zeolite use includes molecular sieves, water purifiers, and catalysts. They may also improve the way oxygen can be removed from air for use in medical & industrial applications.
Zeolite Catalysts: Teaching Models
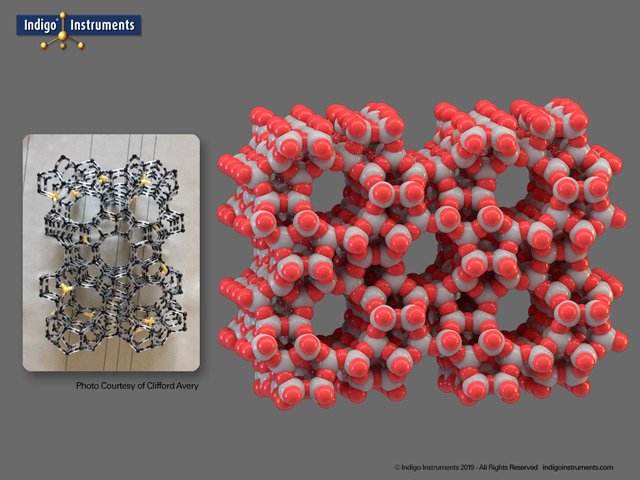
One of our customers, Clifford Avery, uses zeolite molecular models to illustrate how their structure makes them useful for “cracking” hydrocarbons. In other words, reducing crude oil into the various types of fuel we use in heating, cars, barbecues, lamps, and more. Note, that FCC can also mean face-centered cubic which is completely unrelated to this application.
In Cliff’s own words:
“I use the models for Albemarle’s FCC (Fluid Catalytic Cracking) Process Course. In our modules that cover FCC catalysts and FCC additives, these models allow our students to visualize Y-zeolites and ZSM-5 zeolites widely used in the oil refining industry. We can show the supercage sodalite and the side cages. Within the supercage we can show where the acid sites are (green, Al atoms), their distance apart, and how the cracking mechanism occurs. I used the (white) H atoms to demonstrate the process of de-alumination.”

Zeolite Y catalyst built with Orbit scale atoms and bonds. Black & green atoms represent silicon and aluminum respectively. The 4 white hydrogen atoms (marked with*in the image) replace an aluminum atom.
For the ZSM-5 zeolite, we can show how the 2-dimensional pore openings favor the cracking of straight chain olefins. ZSM 5 zeolite catalyst built with Minit atoms/bonds on the left. A computer model is shown to the right (image courtesy Wikipedia).
This structure is quite complex with no instructions available and should only be attempted by an expert. Minit and Orbit are trademarks of Cochranes of Oxford and describe their style of molecular models. They differ only in size; Minit has a 6mm atom centre, Orbit 10mm.
Here is a picture of the modules made with Indigo® Minit molecular model parts.
About Clifford Avery
Clifford Avery is the Global FCC (Fluid Catalytic Cracking)Process Advisor for Albemarle Corporation, Houston, Texas. In this role, he leads the global FCC effort in training, troubleshooting, and performing unit health checks for Albemarle’s FCC customers and sales teams. He has held positions in Business, Technical Services (FCC), and Sales (Hydroprocessing and FCC). His experience in working with FCC units is global, with particular emphasis in the North American and Asia Pacific regions.
Cliff received his B.S. degree in Chemical Engineering from California State University, Long Beach, and has over 33 years of experience in the FCC/hydrocarbon processing industry.
Zeolite Y in 3D
The overall structure of zeolite catalysts can be hard to see on paper or on screen. We photographed our model of Zeolite Y as stereo pairs. One version you can see by crossing your eyes, the other uses the Magic Eye style which requires focusing at a distant imaginary point.
What About Silicon-Based Life?
A month doesn’t go by when some group of astronomers announces it’s found another potentially habitable or Earth-like planet. This sort of hyperbole overlooks the obvious. There is only one Earth. The odds of another world where you can walk off your spaceship, take a deep breath & grab a fruit off a tree is essentially zero. If oxygen-producing organisms had evolved you might not choke on the atmosphere but you’d certainly die of starvation once your food supply ran out.
What about non-carbon-based life, in other words, silicon-based life forms a la Star Trek & others? “Misconceptions of Science: Is Silicon-based Life Possible?” considers the issue. It states that Earth’s crust is made up of 28% silicon which is 1000 times more common than carbon. It goes on to explain key chemical differences between the two elements. In a nutshell, the author concludes that carbon always wins out in the evolution of life.
That is not to say silicon may not have played an important role in the evolution of life on Earth Silicates, such as clay, with their charged surfaces may have acted as support structures analogous to how rocks were used to build bridge arches. Stay tuned for more on this with our next update.